Abstract
Background: Venetoclax (Ven), a highly selective oral BCL-2 inhibitor, in combination with azacitidine (Aza) is approved for the treatment of patients with intensive chemotherapy-ineligible newly diagnosed AML based on the positive results of the phase 3 VIALE-A study (NCT02993523) of placebo (Pbo) vs Ven in combination with Aza in this population (DiNardo et. al. NEJM, 2020). At a median follow-up of 20.5 (range, <0.1-30.7) months (mo), with 270 death events (75% OS) in January 2020, the median overall survival (OS) was 14.7 mo for the Ven+Aza group and 9.6 mo in the Pbo+Aza group (hazard ratio [HR], 0.66; 95% confidence interval [CI], 0.52 to 0.85; P<0.001). Complete remission (CR) was higher with Ven+Aza than with Pbo+Aza (36.7% vs 17.9%; P<0.001), as was complete remission + complete remission with incomplete hematologic recovery (CR+CRi) (66.4% vs 28.3%; P<0.001) (DiNardo et. al. NEJM, 2020). While the study met the statistical significance for its primary endpoint of overall survival at the 75% OS interim analysis in March 2020, with 270 OS events, a 100% final OS analysis was undertaken with 360 survival events (data cut-off of 01 December, 2021), with 2-years of additional follow-up to determine the long-term survival benefit of Ven+Aza. Here, we present the analysis of VIALE-A, after the occurrence of 100% of the pre-planned survival events.
Methods: 431 patients with confirmed AML who were previously untreated and ineligible for intensive therapy were randomly assigned 2:1 to Aza plus either Ven (286 patients) or Pbo (145 patients). All patients received a standard dose of Aza (75 mg/m2 subcutaneously or intravenously on days 1 through 7 every 28-day cycle). Ven 400 mg after a 3 day ramp up to reach the target dose in Cycle 1 or a matching Pbo was administered orally, once daily, in 28-day cycles.
Results: Median age was 76 years in both groups, approximately 60% were male and 76% were Caucasian. Molecular abnormalities of interest included FLT-3, observed in 14.1% of patients receiving VEN+AZA, IDH1/2, observed in 24.9% of pts, TP53, observed in 23.3% of pts and NPM1, observed in 16.6% of pts.
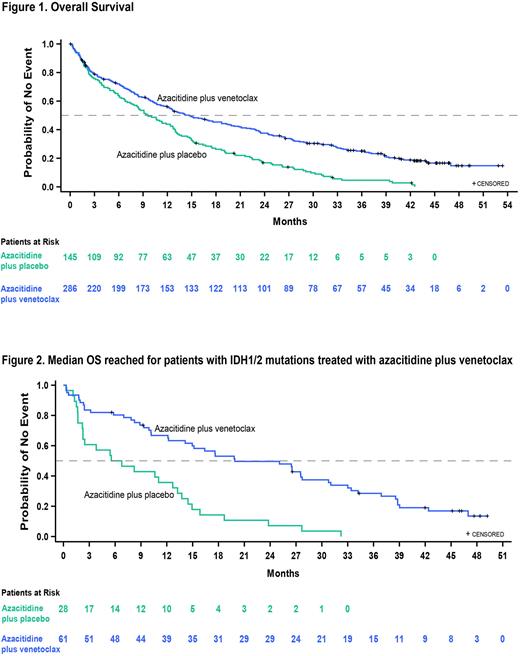
With a median follow-up of 43.2 mo, the median overall survival was 14.7 mo (95% CI, 12.1 to 18.7) in the Ven+Aza group and 9.6 mo (95% CI, 7.4 to 12.7) in the Pbo+Aza group (HR, 0.58; 95% CI, 0.47 to 0.72; nominal P<0.001), maintaining the survival benefit since the interim analysis in the overall population (Figure 1). At this data-cut, 49 pts remained on the study (48 in the Ven+Aza group and 1 in the Pbo+Aza group). In patients with measurable residual disease (MRD) <10-3 who had achieved CR+CRi, median OS was reached at 34.2 mo (95% CI, 27.7-44.0) in the Ven+Aza group (N=69) and 25.0 mo (95% CI, 7.0-39.8) in the Pbo+Aza group (N=11). In CR + CRi patients with MRD >10-3, median OS was 18.7 mo (95% CI, 12.9-23.5) in the Ven+Aza group (N=96) and 15.1 mo (95% CI, 7.4, 26.1) in the Pbo+Aza group (N=24). For patients in the IDH1/2 mutant subgroup, median OS was reached at final analysis, at 19.9 mo (95% CI, 12.2-27.7) in the Ven+Aza group and 6.2 mo (95% CI, 2.3-12.7) in the Pbo+Aza group (HR, 0.314; 95% CI, 0.189 to 0.522; P<0.001) (Figure 2). Overall safety profiles were comparable between Ven+Aza and Pbo+Aza. Key treatment-emergent adverse events of any grade occurring in ≥20% of pts included nausea (44.5% in the Ven+Aza group vs 36.8% in the Pbo+Aza group), diarrhea (45.2% vs 34%), and constipation (43.8% vs 39.6%). Grade 3 or higher AEs (Ven+Aza vs Pbo+Aza) occurring in ≥10% of pts included thrombocytopenia (45.9% vs 39.6%), neutropenia (42.8% vs 28.5%), and febrile neutropenia (42.8% vs 18.8%). Serious adverse events occurred in 85.1% of pts in the Ven+Aza group and 77.1% of those in the Pbo+Aza group. Further characterization of pts with long term benefit is ongoing.
Conclusions: The VIALE-A long-term follow up demonstrates sustained overall survival benefit with Ven+Aza in patients with AML ineligible for intensive chemotherapy compared to Pbo+Aza in all subgroups. Notably, the median OS for patients with MRD <10-3 who had achieved CR+CRi was 34.2 mo, and median OS for patients with IDH1/2 mutations treated with Ven+Aza was 19.9 mo. The VIALE-A 2-year follow-up analysis confirms the long-term survival benefit for patients treated with Ven+Aza, with no new safety findings.
Disclosures
Pratz:AbbVie, Astellas, Boston BioMedical, BMS, Celgene, Novartis, Jazz Pharmaceuticals, and Servier.: Membership on an entity's Board of Directors or advisory committees; AbbVie, Agios, Daiichi Sankyo, Millennium: Research Funding. Jonas:Jazz: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel Reimbursement, Research Funding; BMS: Consultancy, Research Funding; GlycoMimetics: Consultancy, Other: protocol steering committee , Research Funding; Genentech: Consultancy, Research Funding; 47: Research Funding; Gilead: Consultancy, Other: data monitoring committee , Research Funding; Pfizer: Consultancy, Research Funding; Servier: Consultancy; Takeda: Consultancy; Tolero: Consultancy; Treadwell: Consultancy; Accelerated Medical Diagnostics: Research Funding; Amgen: Research Funding; AROG: Research Funding; BMS: Consultancy, Research Funding; Celgene: Research Funding; Daiichi Sankyo: Research Funding; F. Hoffmann-La Roche: Research Funding; Forma: Research Funding; Roche: Research Funding; Hanmi: Research Funding; Immune-Onc: Research Funding; Incyte: Research Funding; Loxo Oncology: Research Funding; LP Therapeutics: Research Funding; Pharmacyclics: Research Funding; Sigma Tau: Research Funding. Pullarkat:AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member. Thirman:AbbVie, AstraZeneca, Celgene,Janssen, Pharmacyclics, Roche/Genentech: Consultancy; AbbVie,Gilead Sciences,Janssen,Merck,Pharmacyclics, Syndax, TG Therapeutics, Tolero Pharmaceuticals.: Consultancy, Research Funding. Garcia:AbbVie, Genentech, AstraZeneca, Prelude and Pfizer: Other: Clinical trial support (institutional) , Research Funding; AbbVie, Astellas, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; AbbVie: Research Funding. Yamamoto:Bristol-Myers Squibb/Celgene: Honoraria, Research Funding; Mundipharma: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Micron/Daiichi-Sankyo: Honoraria; Meiji Seika Pharma: Consultancy, Honoraria, Research Funding; Kyowa Kirin: Honoraria; Takeda: Honoraria, Research Funding; SymBio: Honoraria, Research Funding; Astra-Zeneca: Honoraria, Research Funding; Otsuka Pharmaceutical: Honoraria, Research Funding; Sumitomo Pharma: Honoraria; IQVIA/Incyte: Honoraria; HUYA/IQVIA Services Japan: Honoraria; Novartis: Honoraria; Ono Pharmaceutical: Honoraria; Solasia Pharma: Research Funding; Sanofi: Honoraria; Zenyaku: Research Funding; Yakult: Research Funding; Nippon Shinyaku: Honoraria, Research Funding; MSD: Honoraria; Janssen: Honoraria; Eisai: Honoraria, Research Funding; Astellas: Honoraria; AbbVie: Honoraria, Research Funding; IQVIA/Genmab: Honoraria. Wang:AstraZeneca: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Yoon:Chugai Pharmaceutical: Consultancy; Roche-Genetech: Research Funding; Yuhan Pharmaceutical: Research Funding; Novartis: Consultancy; Kyowa Kirin: Research Funding; Astellas Pharma: Consultancy; Celgene: Consultancy; Janssen Pharmaceutical: Consultancy; Takeda: Consultancy; Amgen: Consultancy; Tikaros: Consultancy. Wolach:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jansen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees. Jang:AbbVie: Research Funding. Yeh:AbbVie: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Chugai Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Janssen Biotech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda Oncology: Membership on an entity's Board of Directors or advisory committees. Ku:Genentech: Current Employment, Current equity holder in publicly-traded company. Zhou:AbbVie: Current Employment, Current equity holder in publicly-traded company. Chyla:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Potluri:AbbVie: Current Employment, Current equity holder in publicly-traded company. DiNardo:Cleave: Research Funding; Astex: Research Funding; LOXO: Research Funding; ImmuneOnc: Honoraria, Research Funding; Takeda: Honoraria; Gilead: Honoraria; Foghorn: Honoraria, Research Funding; Astellas: Honoraria; Forma: Research Funding; Bluebird Bio: Honoraria; Servier: Consultancy, Honoraria, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal